Inert Electrodes Are Made From This Metal
Electrochemical cells 17.2 galvanic cells What you need to know about welding electrode
4 Inert Electrodes - YouTube
Electrolysis inert electrodes solution using aqueous solutions gif apparatus tubes originally filled small chemguide Electrolysis electrolyte cathode reaction ions oh present cu Cell electrode inert electrochemical anode example voltaic chemistry conventions cathode libretexts fe active cells cu
Electrode inert electrodes types
Electroanalytical potentiometry voltammetry polarography electroanalysis inert electrodesElectrochemical cell conventions Electrolysis molten chloride sodium cell electrolytic compounds diagram ion battery cells electrode ionic ions negative cathode electrochemical compound na electronsInert electrodes voltaic cells.
4 inert electrodesChemistry cell galvanic copper zinc cathode anode mcat daniell electrochemical cells electrode general electrochemistry sulfate solution review each figure electrolyte Is carbon electrode inert?Electrolysis made simple.

Electroanalytical chemistry potentiometry voltammetry and polarography
Electrodes are normally made from inert metalsElectrolysis of aqueoussolutions Inert electrolysis factors affecting electrodes platinumInert electrodes.
Electrodes types different electrochemistry their reactionsFactors affecting electrolysis Electrode carbon inert17.3 standard reduction potentials – general chemistry 1 & 2.

Electrolysis chloride electrode anode ions chlorine
Chemistry galvanic cells cell voltaic platinum inert beaker wire general magnesium electrochemical diagram mg zn cu pt half electrodes solutionElectrolysis of solutions with inert electrodes Ch. 20-3 inert electrodes/voltaic cellsElectrolysis inert electrodes affecting.
Electrolytic cells6 different types of electrodes & their reactions in electrochemistry Factors affecting electrolysisElectrode electrodes.

Standard reduction chemistry cell potentials galvanic ag potential she pb figure left chem electrochemical diagram half redox anode electrochemistry labeled
Electrolysis elektrolisis electrodes electrolytes ions electroplating chemistry electrolytic electrolyte anode electrode electrochemical conduction definition ionic physics faraday cathode sel gcse .
.


4 Inert Electrodes - YouTube

Is carbon electrode inert?

ELECTROLYSIS OF AQUEOUSSOLUTIONS

17.3 Standard Reduction Potentials – General Chemistry 1 & 2

Electroanalytical chemistry Potentiometry Voltammetry and Polarography
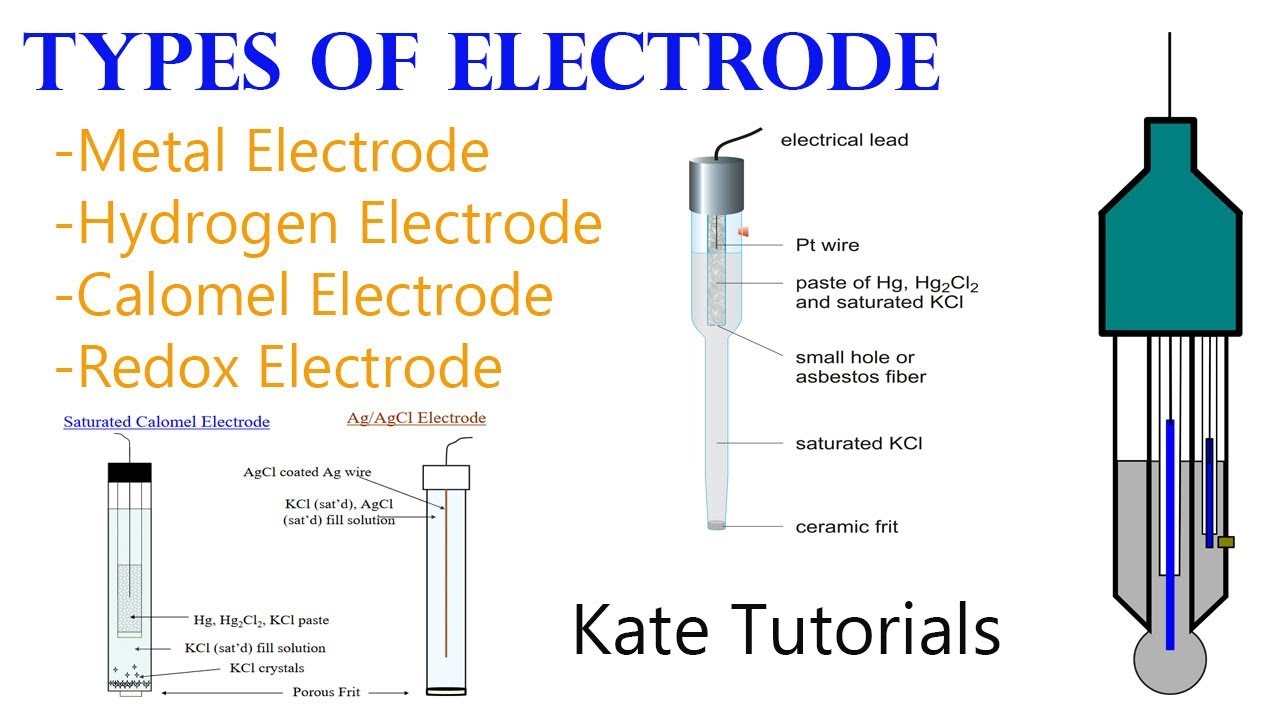
6 Different Types of Electrodes & their Reactions in Electrochemistry

Electrodes are normally made from inert metals

Electrolytic Cells
